Benzyl Alcohol
Benzyl alcohol is bacteriostatic and is used as an antimicrobial preservative against Gram-positive bacteria, molds, fungi, and yeasts, although it possesses only modest bactericidal properties1
Benzyl Alcohol (BA) is a required ingredient in all homebrews.
Benzyl Alcohol can break down2 and evaporate3 with heat. Never heat BA, even in a sealed vial.
BA is a bacteriostatic preservative that stops bacteria from multiplying, but does not kill bacteria.
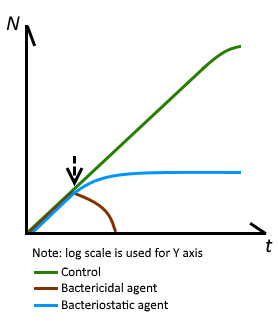
NEVER increase the concentration of BA for ANY reason. Follow a proven recipe closely, one that likely recommends 2% BA. Injecting BA in higher concentrations than what is typically in a vial of HRT, 2%, is potentially dangerous.
Concentrations less than 2% could allow bacteria to proliferate.
Don’t heat it!
Benzyl alcohol is frequently used as an antimicrobial preservative or co-solvent in a varity of pharmaceutical injection formulations. The main toxic oxidation product, benzaldehyde, arises from the oxidation of benzyl alcohol upon long-term storage or heat sterilization of parenteral dosage forms containing benzyl alcohol, if oxygen is not excluded totally by nitrogen flashing. The presence of this potential impurity needs to be monitored owing to its reactivity and toxicity2
This paragraph is in line with the compounding industry’s steady stance that anything containing benzyl alcohol needs to be sterilized using filtration + aseptic technique, and not heat.
Further, benzyl alcohol has a flash point of just 101°C (214°F)3. This means that if you are to ignore the above advice, and you try to sterilize BA through heat, that a significant portion of it is being evaporated. This causes a problem because 1) we don’t want the BA to evaporate, we want it to stay in solution, and 2) if it’s evaporating out of an open container, this is a major flammability hazard.
10.1 Reactivity
Forms explosive mixtures with air on intense heating.
A range from approx. 15 Kelvin below the flash point is to be rated as critical.3
15 Kelvin below the flash point (101°C/214°F) is ~87°C/189°F — this is far below autoclaving temperature (121°C) or dry heat sterilization temperature (160°C).
10.4 Conditions to avoid
…
Strong heating.3
Benzyl alcohol oxidizes slowly in air to benzaldehyde and benzoic acid; it does not react with water… some solutions may generate benzaldehyde during autoclaving.1
So let’s not autoclave it or dry heat sterilize it? Rule #1 of homebrew: Don’t fuck with your benzyl alcohol.
Careful around plastic and rubber
Problems may occur when polystyrene syringes are used with certain types of drug products containing benzyl alcohol since these agents can extract and dissolve the plastic. At times the rubber tip may release a constituent to the drug product.4
Benzyl alcohol can damage polystyrene syringes by extracting some soluble components.1
According to wikipedia, most medical syringes are made of “polyethylene.” I’ve also seen a lot of syringes made of polypropylene on Google. So thinking these polystyrene syringes might not be too common. Regardless, the issues with the rubber tip are universal to the type of plastic in use.
It may, then, be best to measure the BA for your brew using a glass graduated beaker or cylinder so that the 100% BA doesn’t make contact with the rubber. Or, if your BA is in a sterile 100mL vial, to draw from it in a way that minimizes contact with the rubber in the syringe.
Neutralize it for Sterility Assurance Testing
Antimicrobial activity is reduced in the presence of nonionic surfactants, such as polysorbate 801.
If you’re performing sterility assurance testing, BA will interfere as it prevents bacteria from growing. You can use polysorbate 80 to neutralize it. It’s unclear how much is appropriate to use, pending further research.